Tackling Medical Device Usability Design Early Speeds FDA Approval
For medical device makers, it’s best to embrace the FDA regulatory process early as substantial time and effort is needed to prepare for approval. The FDA requires device makers to prove through appropriate testing that use errors are greatly reduced and that a device does not allow use errors to cause harm to a patient.
The more risk that a device poses to a patient, the more rigorous the approvals process and required testing. The regulatory process can take as little as 90 days for a low-risk Class II device, such as a blood pressure cuff, to as much as 12+ months for a complex, high-risk Class III device like an implantable pacemaker.
For this reason, doing usability design up front in your product creation process can prove to be very economical in terms of both time and cost. Use-errors found in summative or late-stage testing can force changes that would have been avoided by doing usability design at the outset.
Daunting Process Worth the Effort
This process can seem daunting and annoying to stakeholders who aren’t practiced in usability design and testing. But for usability designers, such as Human Factors Engineers and UX Designers who are user- (and patient) focused, it makes good sense.
In fact, most usability designers probably wonder what took the FDA so long to regulate medical device safety at the level they do now, considering the number of errors and the amount of patient harm reported from use errors over the last few decades. For example, the FDA reported that between 2005 and 2009 drug-infusion pumps and related devices accounted for 35% of medical errors that resulted in significant patient harm. The agency said a large number of the adverse events stemmed from "programming errors attributed to poor device usability." Essentially, that's human error arising from a poorly designed UI.
But, where the FDA regulatory process may seem troublesome to stakeholders, for usability designers it is just a more rigorous and well-documented version of their typical best practice, user-centered design process.
All FDA submissions have a legal requirement for a Quality System or current good manufacturing practices, which in turn requires Design Controls or the application of a formal methodology, so everyone is mandated to have a design process. What that process is and how it is handled makes a big difference to your final product. Following a best practice, user-centered design process is an efficient and effective way to set yourself up for success with an FDA approval.
Scrimping Now May Cost you Later
As the stakeholder, you may think it’s OK to skip doing a usability design as a way to save time and money, assuming that developers can fill in the UI as they program the device, and/or that you will deal with usability as an add-on once you begin testing.
Please think again.
Ending up with a design by default or accident and being forced to make major design changes in the midst of your testing process will cause you more headaches and delays in your FDA approval process. You will be discovering in your summative testing that you have multiple problems that you need to fix. You’ll spend more time and money trying to fix at the end what you could have — should have — done at the beginning.
Design, once past the brainstorming phase, is an orderly process. After articulating the requirements, context of use and user stories, designers will design to the user stories. Then they’ll evaluate their designs against the requirements and context of use.
This is an iterative process of design/evaluate/modify, repeated until the design satisfies all the requirements. At that point, and sometimes sooner, designers will test the design with actual users. In the language of the FDA requirements, these are formative tests. At the end of that process, you can be assured that you have a well-designed product that will likely sail pretty smoothly through the summative tests needed for your approval documents.
Here is a simple roadmap of the design inputs and outputs of a usability design process:
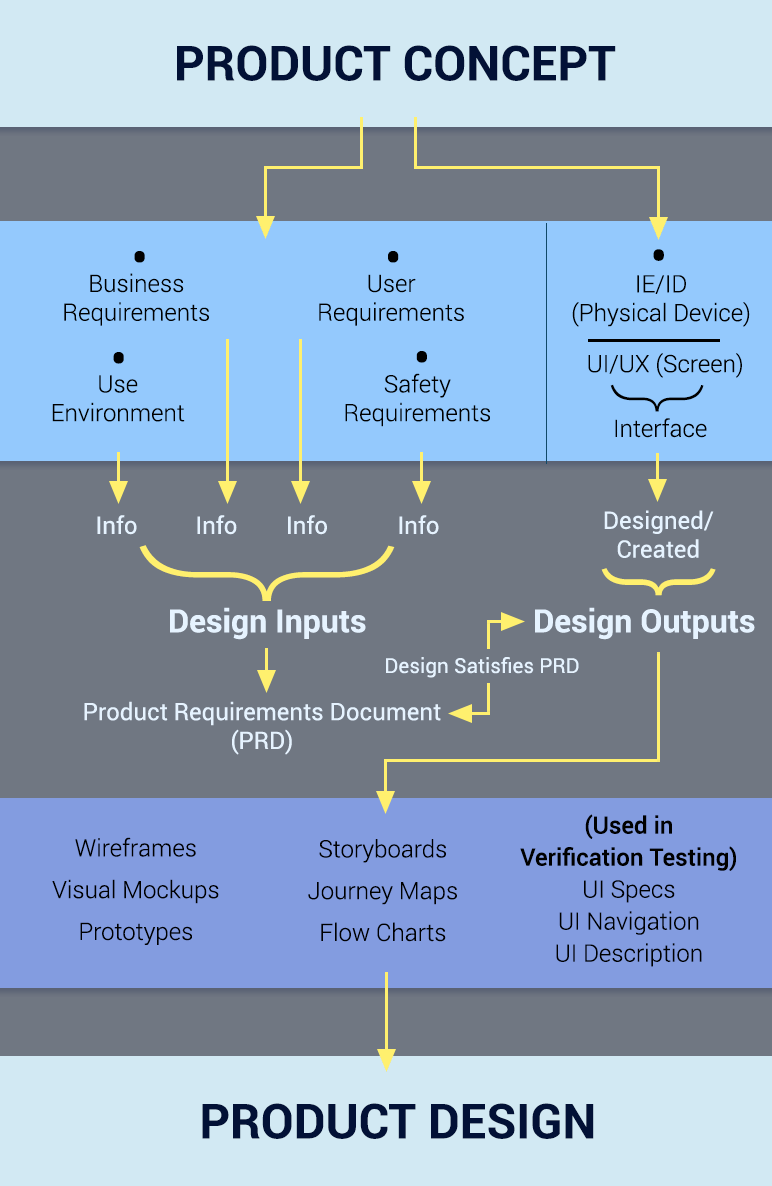
So what am I advising? Start usability design at the beginning of your project, as soon as you can articulate your business, user and safety requirements and know how and where your device will be used (context of use). This establishes a strong foundation for a safe, usable device that is well-positioned to gain FDA approval without undo cost overruns and schedule changes. If your wait to do usability design, your product launch may be delayed as you answer FDA questions.
How much does that delay cost your company?
For more on medical device usability, download our ebook Designing Better Medical Devices: Tips on Usability, Safety and Compliance.