AI-Powered
Threat Modeling
Accelerate Cybersecurity Risk Management for FDA Submissions with Aithra
Unlock the future of medical device compliance with AI-powered Automation for Threat Modeling and Risk Assessment (Aithra, pronounced eye-thra). This revolutionary tool developed by our partner BG Networks is specifically built for medical device manufacturers. Leveraging advanced Large Language Models (LLMs), Aithra makes it easier and faster—up to 10x quicker—to generate reliable, FDA-compliant cybersecurity risk assessments.
Why Aithra?
The FDA's Secure Product Development Framework (SPDF) requires a detailed, risk-based approach from design to end-of-life. Aithra simplifies this process by automatically generating the required assessments in minutes, providing a streamlined report in an easy-to-export format. This ensures that your submission is ready to integrate with your SPDF documents and submit to the FDA without the hassle. With Aithra you can expect:
10x Efficiency Gains
Speed up risk analysis and documentation by automating complex workflows, saving time and effort.
Early Design Risk Assessment
Get early insights into security risks and necessary controls as soon as your system description is ready, helping you identify vulnerabilities before they become issues.
Dynamic Risk Updates
Stay compliant with evolving threats by continuously updating your risk assessments as new threats emerge.
Boost Efficiency and Compliance with Aithra
Whether you're a large company with established processes or a smaller company navigating your first FDA submission without a dedicated cybersecurity team, Aithra ensures you stay ahead of the compliance curve with minimal effort. How it works in 3 simple steps:
Upload your device documentation
Gather your existing medical device files, diagrams, and PDFs.
Submit to Aithra
Upload your documents to the platform.
Generate your report
Aithra instantly produces a fully FDA-compliant risk and threat assessment report.
System Documentation
Based on industry standards recognized by FDA: TIR57, ANSI/AMII SW96, ISO 800-30 & IEC 81001-5-1.
TheatRiskAnalysis.xlsx
See AITHRA in Action!
Access our on-demand threat modeling webinar where we go step-by-step through a threat model and risk assessment for a number of attack paths and show you how it's done with Aithra.
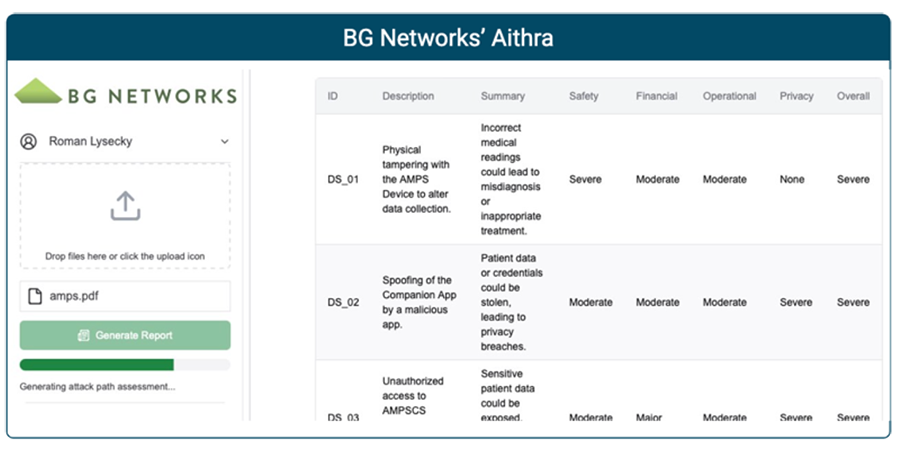
Aithra’s Risk Assessment Process
Aithra generates comprehensive risk assessments through a systematic analysis process.
Asset Identification
Define key system components and data assets
Threat Modeling (STRIDE)
Identify potential threats using the STRIDE framework
Risk Assessment
Prioritize risks and determine necessary security controls

Damage Scenario
Assess the potential impact of security breaches
Attack Likelihood Analysis
Evaluate the probability of each attack scenario
Attack Path Enumeration
Map out possible attack vectors and entry points